We have used microscopes to help us discover microscopic phenomena for hundreds of years. Thanks to the digital image sensor and computer, much of this discovery work has now started to become automated. A variety of machine learning algorithms now automatically process digital microscope images to find, classify and interpret relevant phenomena. Examples include searching for defects within electronic devices, checking for the presence of certain cells in an assay, or examining biological samples to search for indications of disease.
Despite their automation, microscopes have still changed relatively little - they are, for the most part, still optimized for a human viewer to peer through to examine a sample in detail. With infectious disease diagnosis, for example, human-based analysis of light microscope images is still the diagnostic gold standard. The diagnosis of infection by the malaria parasite offers a good example of the challenges surrounding light microscopy in the clinic. Due to their small size (approximately 1 micron or less), the malaria parasite (P. Falciparum) must be viewed under a high-resolution objective lens, typically using oil immersion. Unfortunately, it is challenging to create such high-resolution lenses that can image over a large area. As the infection density of the malaria parasite is relatively low, a trained professional must scan through several hundred unique fields-of-view to find enough examples to offer a sound diagnosis. This leads to a bottleneck in the diagnosis pipeline - inspection of a typical sample takes 10 minutes or more.
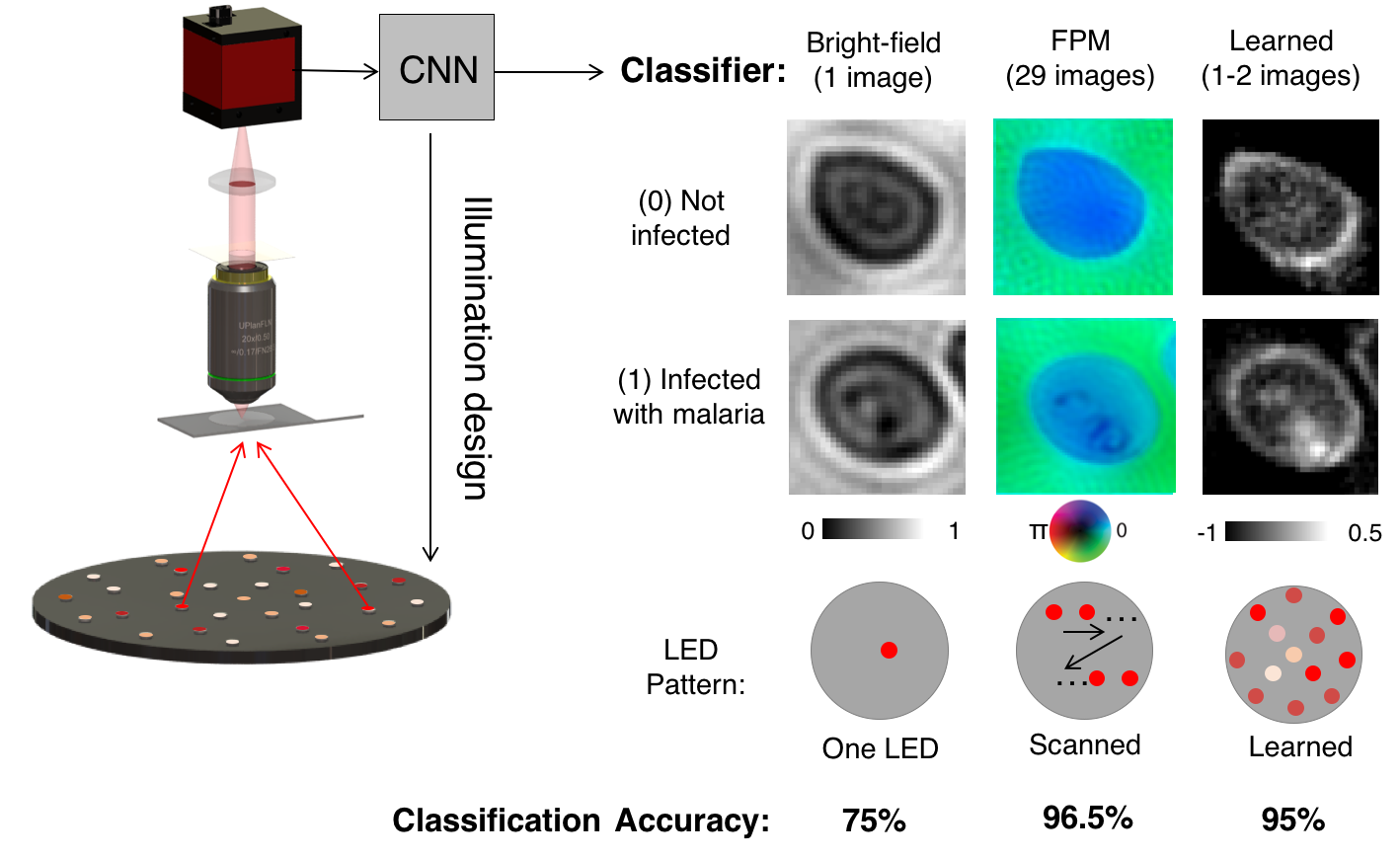
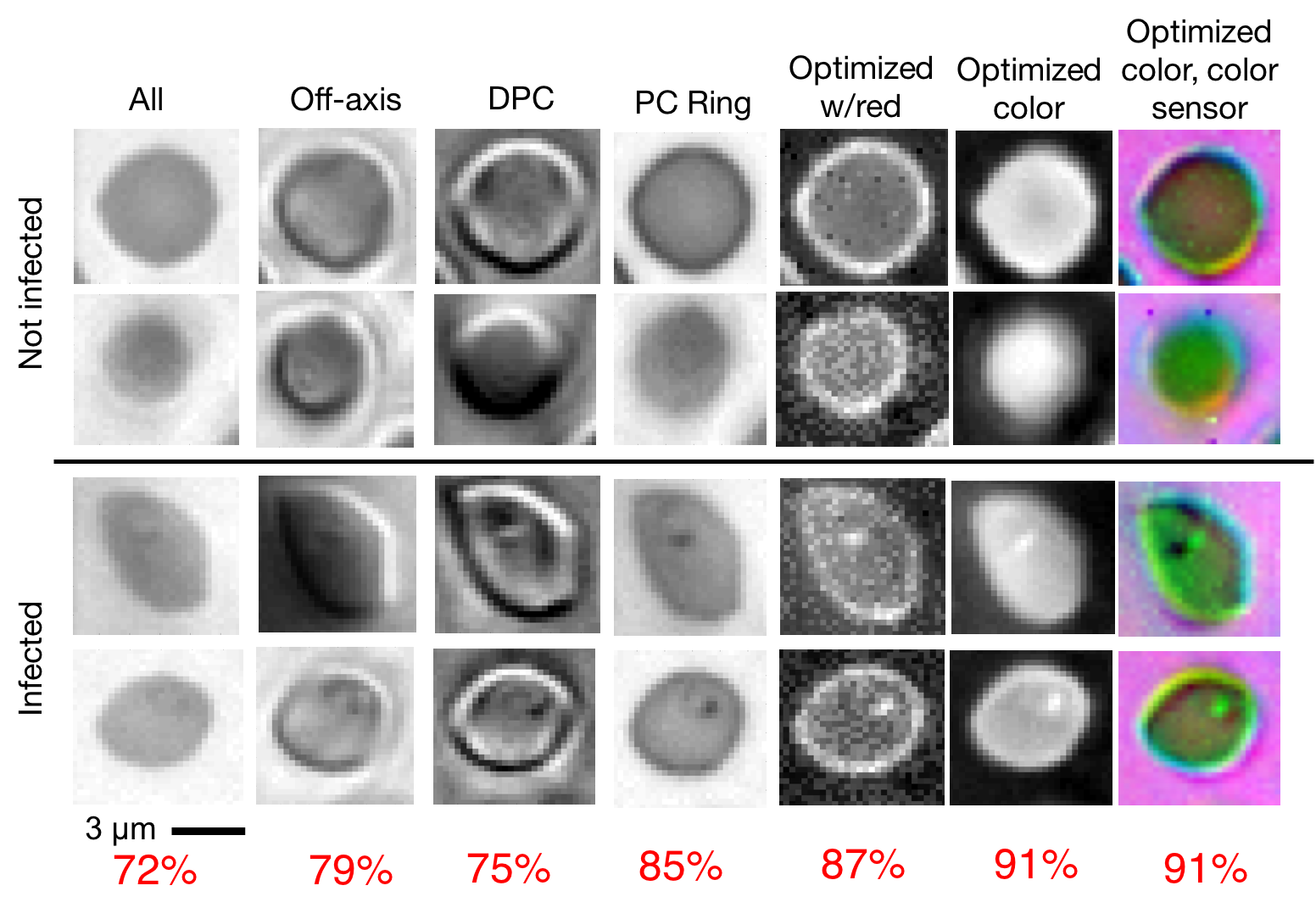
Here, we suggest an approach that can significantly improve the speed and accuracy of disease diagnosis via light microscopy. Our approach is based upon two key modifications to the standard microscope: 1) the addition of a micro-LED unit that is optimized to illuminate each sample (e.g., blood or sputum smears) to highlight important features of interest, and 2) the adoption of a deep neural network that is jointly optimized to automatically detect the presence of infection within the uniquely illuminated images. Working together, our two insights allow us to achieve classification accuracies in the 95 percentile range using large field-of-view, low-resolution microscope objective lenses that can see thousands of cells simultaneously (as opposed to just dozens of cells). This removes the need for mechanical scanning to obtain an accurate diagnosis, subsequently offering a dramatic speedup to the current diagnosis pipeline (i.e., from 10 minutes for manual inspection to just a few seconds for automatic inspection).
If you would like additional information about our approach to re-design the microscope to be better at automated disease diagnosis, please feel free to get in touch!